Abstract
Introduction
One of the emerging targeted strategies for treatment of multiple myeloma (MM) is the use of robust immune responses via T cell activation against tumor cells. B-Cell Maturation Antigen (BCMA), a cell surface protein, remains a potential target for therapeutic interventions. Recently, bispecific T cell engager therapy, targeting BCMA and CD3 antigens on plasma and T cells respectively, have shown promising results in preclinical and early clinical studies in the context of relapsed/refractory myeloma (RRMM). We conducted a systematic review of phase 1 trials to report the efficacy and safety of Bispecific T-Cell Engager antibodies for MM.
Methodology:
We systematically searched multiple databases, including PubMed, Embase, Cochrane, and Clinicaltrials.gov. We also searched major conferences for oral or poster presentations. MeSH terms and keywords for MM and bispecific antibodies were utilized. We included all original studies reported in English language published from 1990 until June 2021. The primary database search yielded 390 articles. After excluding review articles, duplicates, irrelevant articles, and non-human studies, six phase 1 clinical trials were included to evaluate the efficacy and safety outcomes.
Results:
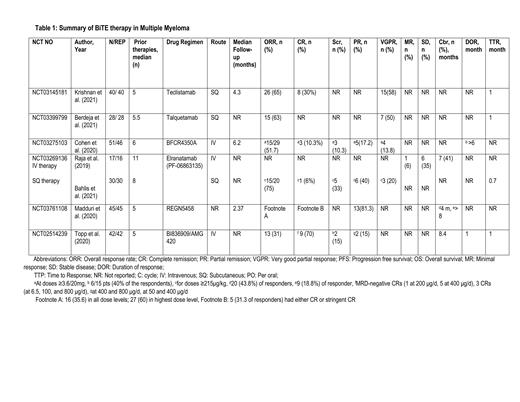
A total of 253 patients were identified from the six phase 1 studies, among whom 247 were evaluable for response, while the safely analysis involved all the patients. A study conducted by Krishan et al. (n= 40) stated an overall response rate (ORR) of 65% among patients receiving subcutaneous (SQ) teclistamab with a median of five prior therapies, 30% and 58% of the responders had a complete response (CR) and very good partial response (VGPR), respectively. A similar ORR of 63% was reported in 28 patients with a median of 5.5 prior therapies receiving SQ talquetamab by Berdeja et al. Cohen et al reported an ORR of 51.7% in patients receiving IV BFCR4350A at doses ≥3.6/20mg (Partial response (PR):17.2% and CR: 10.3%). SQ elranatamab achieved an ORR of 75% in 17 patients who received doses ≥215 μg/kg. Madduri et al. evaluated REGN5458 monotherapy with dose escalation from 3 to 96 mg among 45 patients and reported an ORR of 60% at the highest dose levels. ORR of 31% was reported in 42 patients receiving IV BI836909/AMG 420 by Topp et al. The median time to response (TTP) was 1 month in the majority of the studies. (Table 1)
The most common grade three or higher hematological toxicities were neutropenia and anemia, while among non-hematological toxicities, infections were the most common. The incidence of cytokine release syndrome and neurotoxicity varied from 24% to 74% and 2 % to 20%, respectively. (Table 3)
Conclusion:
The early clinical data of Bispecific T-Cell Engager therapy in heavily pretreated RRMM patients shows promising results regarding its efficacy and safety profile. It represents a whole new horizon of targeted approach towards RRMM. Phase II / III studies are being conducted to evaluate this potential treatment approach in patients with MM.
Anwer: GlaxoSmithKline: Research Funding; BMS / Celgene: Honoraria, Research Funding; Allogene Therapeutics: Research Funding; Janssen pharmaceutical: Honoraria, Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal